
Estimation of measurement uncertainty and validation of RP-HPLC for simultaneous determination of five antihistamines in pharmaceutical formulations | SpringerLink

Development and Validation of Reversed-Phase High Performance Liquid Chromatographic Method for Hydroxychloroquine Sulphate. - Abstract - Europe PMC

PDF) Development of UV Spectroscopic Method for Nefopam and Escitalopram as INN Drugs in Tablet Dosage Form | Zakiur Rahman and Kanij Fatema - Academia.edu

pronunciación maratón Suave the interday and intraday assay repeatability was less than - happilyhomeschooling.com

Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics

pronunciación maratón Suave the interday and intraday assay repeatability was less than - happilyhomeschooling.com

Concentration Determination of >200 Proteins in Dried Blood Spots for Biomarker Discovery and Validation - ScienceDirect
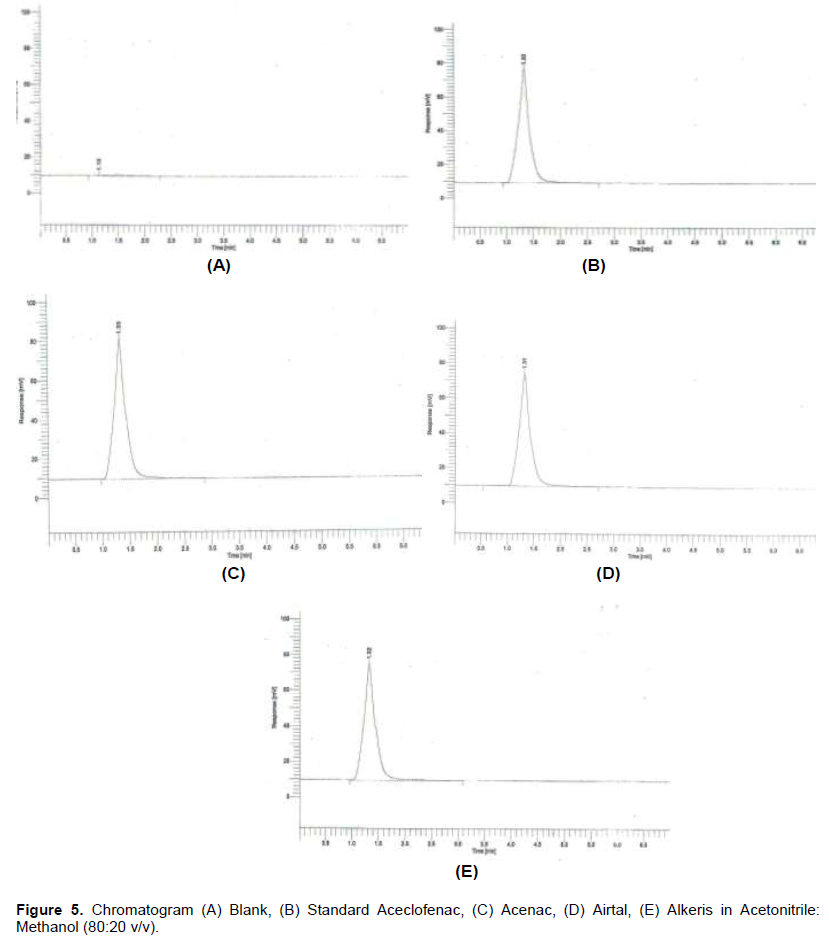
African Journal of Pharmacy and Pharmacology - development and validation of uv- spectrophotometric and rp-hplc method for the analysis of raw material and formulations of aceclofenac
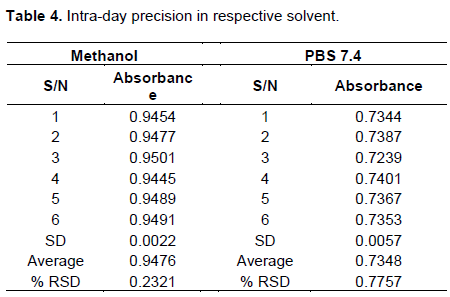
African Journal of Pharmacy and Pharmacology - development and validation of uv- spectrophotometric and rp-hplc method for the analysis of raw material and formulations of aceclofenac

Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics

Development of Micellar Electro Kinetic Chromatography for the Separation and Quantitation of L-valine, L-leucine, L-isoleucin and L-phenylalanine in Human Plasma and Comparison with HPLC - SciAlert Responsive Version

Development and validation of rp-hplc method for estimation of febuxostat in tablet dosage form on a kanak column
Comparative Analysis of Protein Quantification Methods for the Rapid Determination of Protein Loading in Liposomal Formulations
Development and validation of a stability indicating HPLC method for the simultaneous analysis of lopinavir and ritonavir in fix

pronunciación maratón Suave the interday and intraday assay repeatability was less than - happilyhomeschooling.com
A New Rapid and Sensitive Stability-Indicating UPLC Assay Method for Tolterodine Tartrate: Application in Pharmaceuticals, Human





![Full text] Comparative study of β-cyclodextrin, γ-cyclodextrin and 4-t | DDDT Full text] Comparative study of β-cyclodextrin, γ-cyclodextrin and 4-t | DDDT](https://www.dovepress.com/cr_data/article_fulltext/s201000/201907/img/DDDT_A_201907_T0004.jpg)